Official announcement! Five pharmaceutical companies in China obtained the license for free imitation and production of Pfizer COVID-19 oral medicine.
The market rumors of Pfizer COVID-19’s oral medicine imitation license have been confirmed.
On March 17th, official website, a pharmaceutical patent pool (MPP), announced that it had signed agreements with 35 companies, which were allowed to copy and produce nirmatrelvir, one of the ingredients of Paxlovid, an oral drug of Pfizer COVID-19.
According to the map released by official website, the countries involved in the agreement are distributed in 12 countries around the world, among which 6 companies will focus on the production of APIs, 9 companies will produce drugs, and the remaining 20 companies will have both. There are five pharmaceutical companies in China, including Huahai Pharmaceutical (600521), Puluo Pharmaceutical (000739), Fosun Pharmaceutical (600196), Jiuzhou Pharmaceutical (603456) and Shanghai Disino, among which Jiuzhou Pharmaceutical only produces APIs, while others can produce APIs and preparations at the same time.
It is worth noting that the agreement will help to expand the accessibility of Pfizer COVID-19 oral medicine in 95 low-and middle-income countries, accounting for about 53% of the world population, but excluding China.
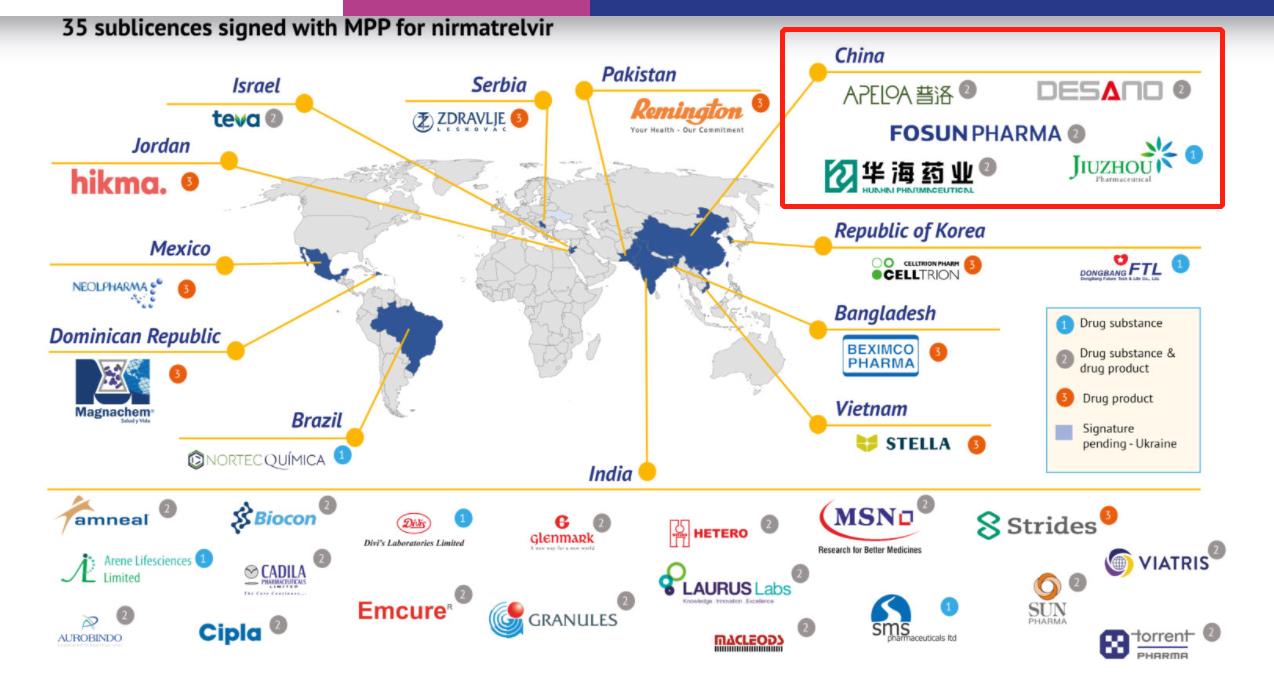
Earlier on March 17, the above news was circulated in the market, and the above listed companies once had a daily limit. Previously, it was rumored in the market that Puluo Pharmaceutical could only produce APIs. According to the newly published map, Puluo Pharmaceutical can produce both raw materials and preparations.
It is worth noting that a Ukrainian company is also on the list of 35 companies. MPP said that due to the current conflict, it could not be signed, but the license could still be used.
According to the data of official website, the Pharmaceutical Patent Pool Organization is a public health organization supported by the United Nations, which is committed to increasing access to life-saving drugs for low-and middle-income countries and promoting drug development.
On January 20th, local time, the Pharmaceutical Patent Pool Organization (MPP) announced through official website that it had signed an agreement with 27 pharmaceutical companies to allow them to produce and supply the oral anti-Covid-19 drug Molnupiravir of Merck to 105 low-and middle-income countries or regions around the world, so as to promote the affordability and accessibility of the drug in the world. Five China pharmaceutical companies, such as Fosun Pharma, Borui Pharma, Shijiazhuang Long Ze Pharmaceuticals, Shanghai Diseno and Langhua Pharmaceuticals, are among them, of which the first four are allowed to produce both raw materials and finished products, and Langhua Pharmaceuticals is allowed to produce raw materials.
In other words, two pharmaceutical companies, Fosun Pharma and Shanghai Disino, have obtained the rights to prevent and control the production of Pfizer and Merck.
Pfizer’s Paxlovid is a combination of PF-07321332 and ritonavir, which has been approved for conditional marketing in China. Molnupiravir of Merck is a nucleoside drug in oral form, which has not been approved in China.