The latest inventory of innovative drugs of traditional Chinese medicine in 2022! Innovative Chinese medicine ushers in spring
Under the influence of the reform of drug evaluation and approval system and multiple favorable factors, the process of listing new drugs in China has been accelerated, and local innovative pharmaceutical companies have begun to rise. Although chemical drugs and biological products have occupied the mainstream of the market, with the continuous implementation of the support policy for innovative drugs of traditional Chinese medicine, innovative research and development of traditional Chinese medicine has also made breakthrough progress, stepping on the wave of the times to usher in spring.
According to National Medical Products Administration’s "Drug Evaluation Report for 2021", 34 pieces of traditional Chinese medicines were approved in 2021, up by 21.43% year-on-year, including 28 pieces (28 varieties) of innovative traditional Chinese medicines, up by 16.67% year-on-year; It is recommended to approve 14 pieces of NDA, with a year-on-year increase of 250.00%, a new high in five years, including 11 pieces of NDA (11 varieties), with a year-on-year increase of 175.00%.
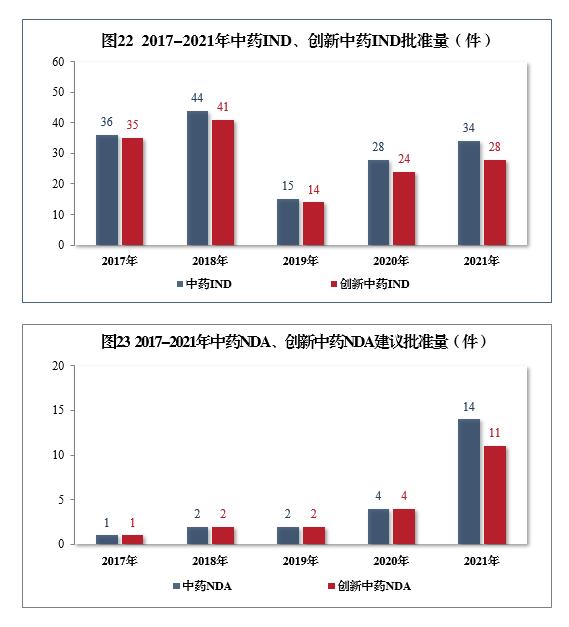
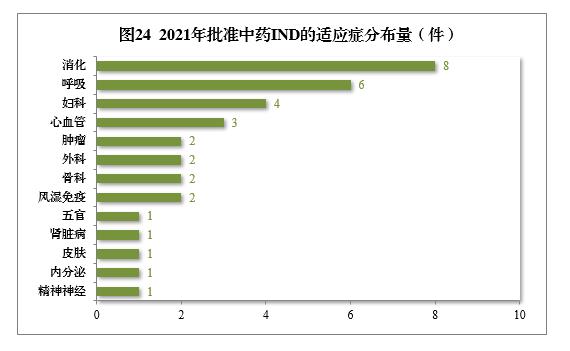
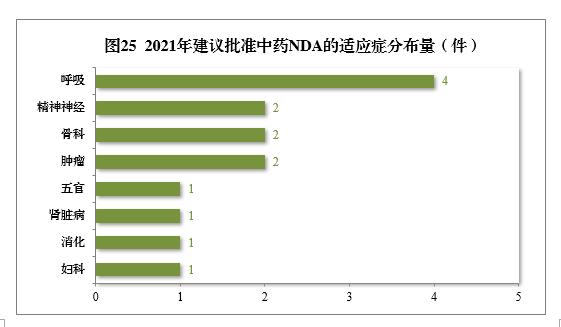
Among the 34 pieces of traditional Chinese medicine IND approved, 13 indications were involved, including 8 pieces of digestion, 6 pieces of respiration and 4 pieces of gynecology, accounting for 52.94%. Among the 14 pieces of NDA recommended for approval, respiratory tumor, mental nerve and orthopedics drugs are more, accounting for 71.43% of all NDA approvals.
In 2021, the innovative drugs of traditional Chinese medicine approved for listing are:
Yiqi Tongqiao PillIt has the effects of invigorating qi, consolidating exterior, dispelling wind and inducing resuscitation, and is suitable for treating seasonal allergic rhinitis which belongs to deficiency of lung and spleen according to TCM syndrome differentiation. This product is a compound preparation of 6 kinds of traditional Chinese medicines, which consists of 14 kinds of herbs, such as Radix Astragali and Radix Saposhnikoviae. Tianjin Dongfang Huakang Pharmaceutical Technology Development Co., Ltd. is the holder of the drug listing license for this variety.
Yishen Yangxin Anshen tabletsIts main functions are tonifying kidney, nourishing heart and calming nerves. It is suitable for treating insomnia, which is characterized by heart-blood deficiency and kidney essence deficiency. The symptoms are insomnia, dreaminess, palpitation, listlessness, forgetfulness, dizziness, soreness and weakness in the waist and knees, pale red tongue with thin white fur, and deep or thin pulse. This product is a compound preparation of six kinds of traditional Chinese medicines, which consists of 10 kinds of herbs, such as fried Zizyphus jujuba seeds and prepared Polygonum multiflorum Thunb.
Information of Drug Approval for Yishen Yangxin Anshen Tablets (Part)
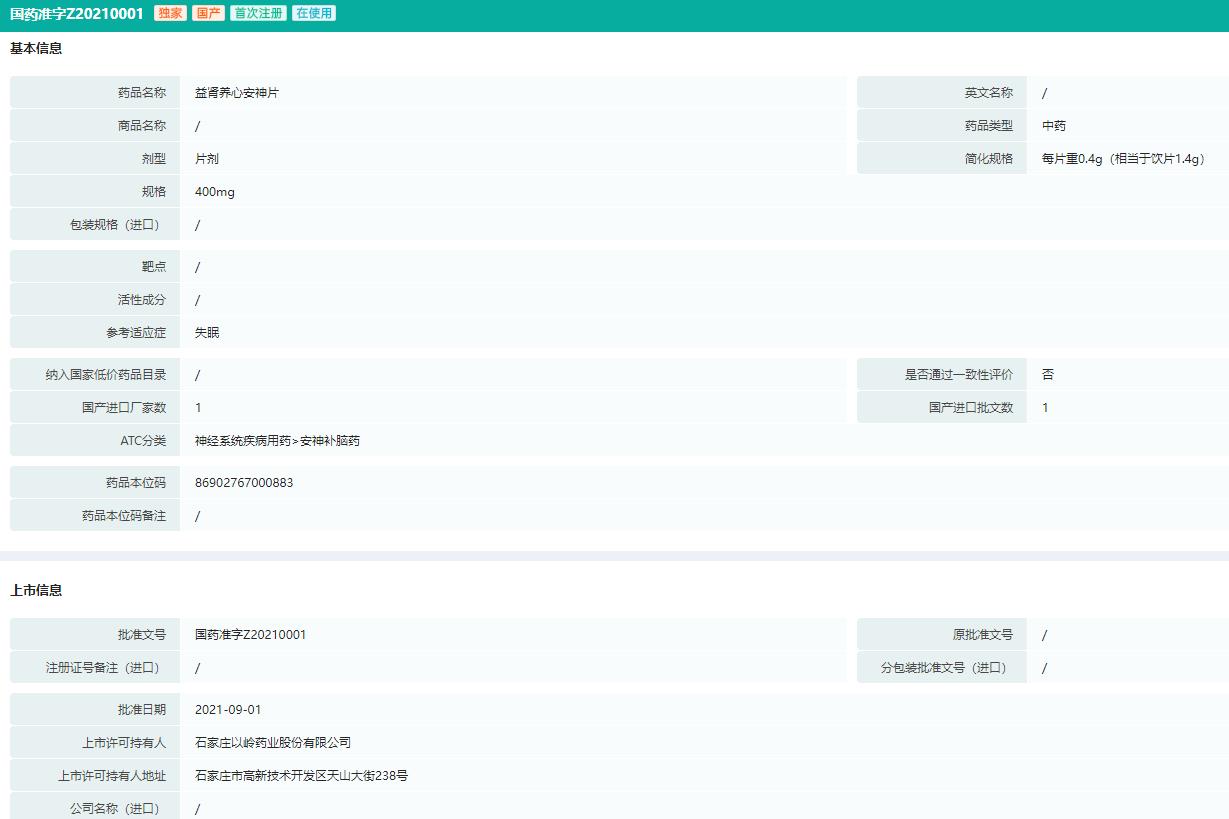
Image source: Rong Yun China Drug Approval Database.
Yinqiao Qingre tabletsIndications are pungent cooling, relieving exterior syndrome, clearing away heat and toxic materials. It is suitable for treating common cold of exogenous wind-heat type, with symptoms of fever, sore throat, foul wind, stuffy nose, runny nose, headache, body aches, sweating, cough, dry mouth, red tongue and rapid pulse. This product is a 1.1-class innovative Chinese medicine composed of 9 kinds of herbs, such as honeysuckle and pueraria lobata.
Xuanqi Jiangu TabletIt has the effects of promoting blood circulation, relaxing muscles and tendons, dredging meridians, relieving pain, tonifying kidney and strengthening bones, and is suitable for the treatment of mild and moderate knee osteoarthritis, which belongs to the symptom improvement of tendon and blood stasis syndrome in TCM. This product is a 1.1-class innovative Chinese medicine composed of 11 kinds of herbs, such as Corydalis yanhusuo and Scorpio.
Qizhi Yishen capsuleIt has the effects of benefiting qi and nourishing yin, removing blood stasis and dredging collaterals, and is suitable for the treatment of deficiency of both qi and yin in early nephropathy. This product is a 1.1-class innovative Chinese medicine composed of 10 kinds of herbs, such as Radix Astragali and Radix Rehmanniae.
Kunxinning granulesIt has the effects of warming yang, nourishing yin, benefiting kidney and calming liver, and is suitable for treating female climacteric syndrome, which belongs to deficiency of both kidney and yin. This product is a 1.1-class innovative Chinese medicine composed of 7 kinds of herbs, such as Rehmannia glutinosa and Concha Haliotidis.
Patent Information of Kunxinning Granules (Part)
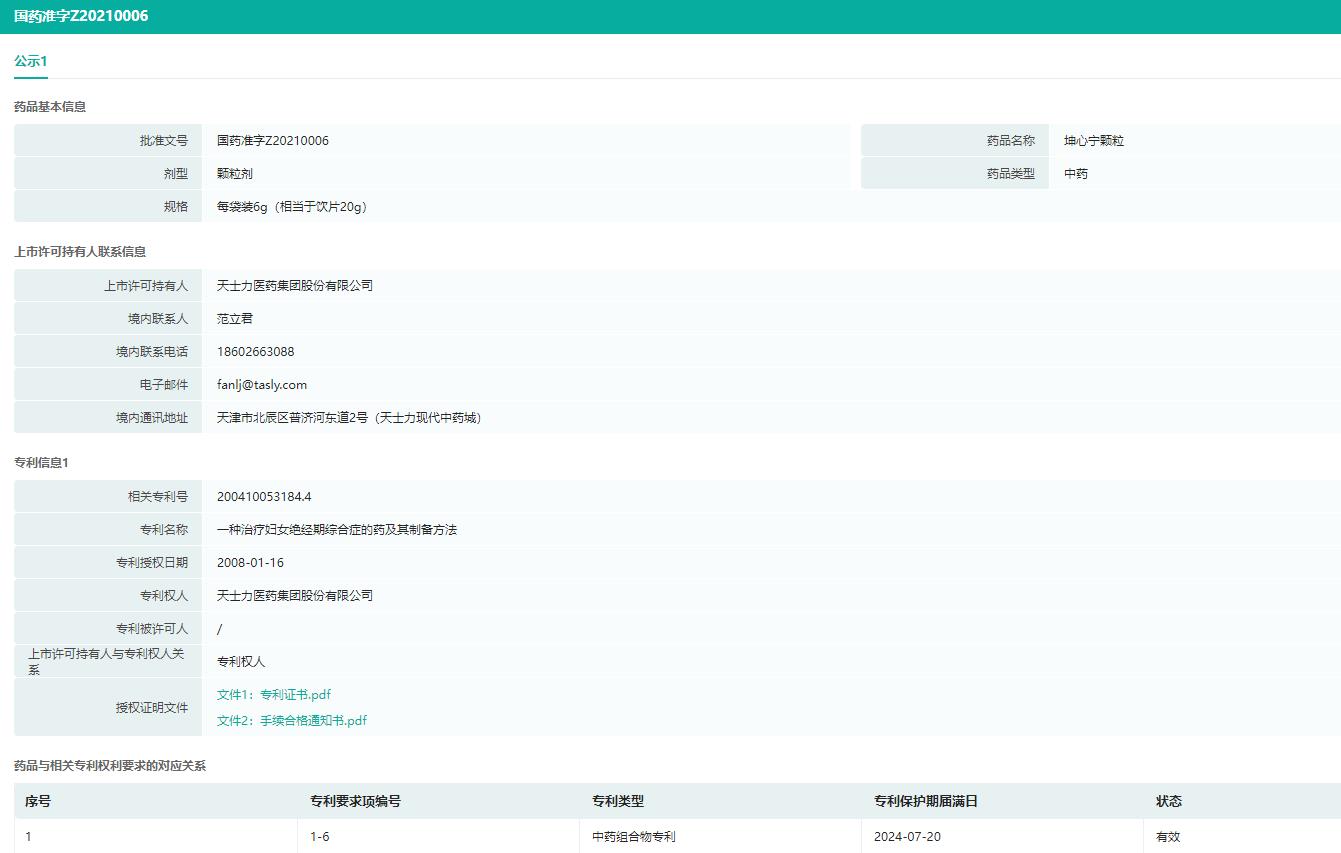
Image source: Rong Yun China listed drug patent database.
Huzhen Qingfeng capsuleIt has the effects of clearing away heat and promoting diuresis, removing blood stasis and promoting turbidity, nourishing liver and kidney, and is suitable for treating mild to moderate acute gouty arthritis, which belongs to damp-heat accumulation syndrome according to TCM syndrome differentiation. This product is a 1.1-class innovative Chinese medicine composed of four kinds of herbs, such as Polygonum cuspidatum and Plantago asiatica.
Jieyu Chufan CapsuleIt has the effects of relieving depression, resolving phlegm, clearing away heat and relieving annoyance, and is suitable for treating mild and moderate depression, which belongs to the syndrome of stagnation of qi and phlegm and stagnation of fire. This variety is a 1.1-class innovative Chinese medicine composed of 8 kinds of herbs, such as Gardenia and Ginger Magnolia officinalis. It is developed on the basis of clinical experience of traditional Chinese medicine, and the prescription is based on Banxia Houpu decoction recorded in the classic Chinese medicine book synopsis of the golden chamber and Gardenia Houpu decoction recorded in Treatise on Febrile Diseases.
Icariin soft capsulesIt is suitable for the treatment of unresectable hepatocellular carcinoma, which is not suitable or the patient refuses to receive standard treatment and has not received systemic treatment before. The composite markers of patients’ peripheral blood meet at least two of the following detection indexes: AFP ≥ 400 ng/ml; TNF-α<2.5pg/mL; IFN-γ≥7.0pg/mL。 This is the first small molecular immunomodulator from Epimedium in China.
Qirui Weishu CapsuleIt has the effects of promoting blood circulation, removing blood stasis, eliminating dampness and relieving pain, and is suitable for the treatment of epigastric pain caused by mild to moderate chronic non-atrophic gastritis with erosion, damp heat and blood stasis. This product is a 1.1-class innovative Chinese medicine composed of four kinds of herbs, such as Panax notoginseng and dried alum. Qirui Weishu Capsule took 375 days from the application for listing to the approval of production by the Drug Examination Center. (If you want to know the evaluation progress of the target product in real time, you can subscribe to the product on the official website of Rong Yun Pharmaceutical Co., Ltd. to help you know the latest situation of potential competitive products, predict the time of listing of competing products, adjust the product sales strategy in time, avoid risks, and register for a 15-day free trial! )
Timeline of Qirui Weishu Capsule Review (Part)
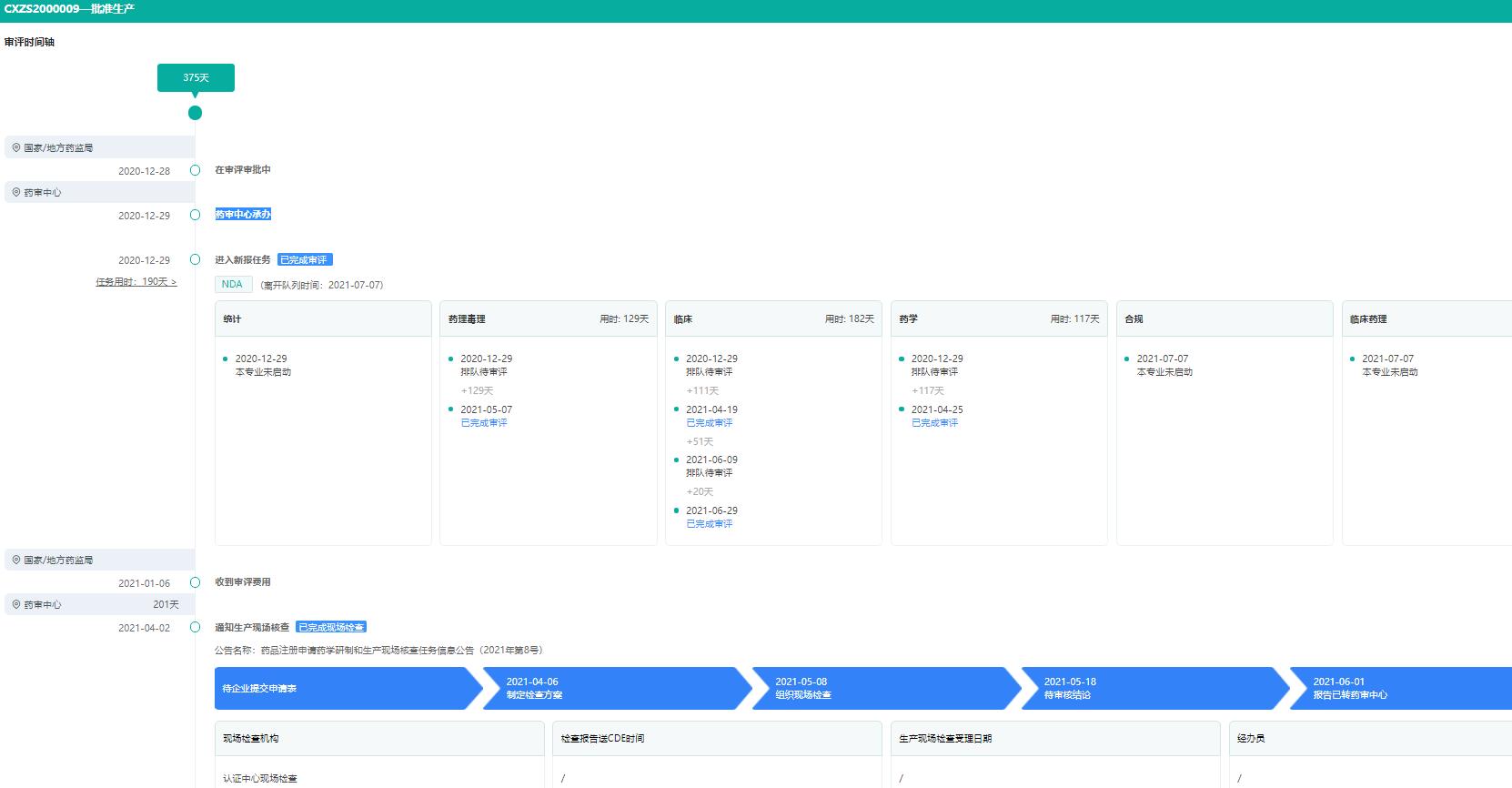
Image source: Rong Yun China Drug Evaluation Database.
It can be seen that the approval of innovative drugs of traditional Chinese medicine is accelerating. According to historical data, in 2017, it even took 3,600 days to approve an innovative Chinese medicine, which was slightly shortened in 2018, less than 2,000 days. In 2021, the accelerated trend of evaluation became more obvious, and it was approved for listing in about 300 days, and the time to market was shortened by 90%! And a new anticancer drug approved for marketing in 2022.Icariin soft capsulesIt took less than 300 days to be approved for listing.
The data of innovation registration and evaluation of traditional Chinese medicine is refreshed again, which reflects the continuous deepening of the reform of drug evaluation and approval system, encourages the continuous overweight of innovation policy dividends, and further improves the evaluation ability and efficiency.
According to the latest data of CDE official website, as of September 30th, 2022, there were 49 varieties of innovative Chinese medicine accepted and 15 varieties under trial, namelyCompound Chrysanthemum Indicum Flavonoids Granules、Kangmin Zhenke Granule、Zhike Juhong Granule、Zhengqingfengtongning sustained-release tablets、AC591 particles、Xinyang tablet、Xiaoer Elsholtzia granules、Prostaglandin suppository、Chaiqin Tonglin tablets、Shenwei Ningyu tablets. It remains to be seen whether the innovative Chinese medicine drugs approved for marketing in 2022 will reach a new high.
List of innovative Chinese medicine varieties under review in 2022
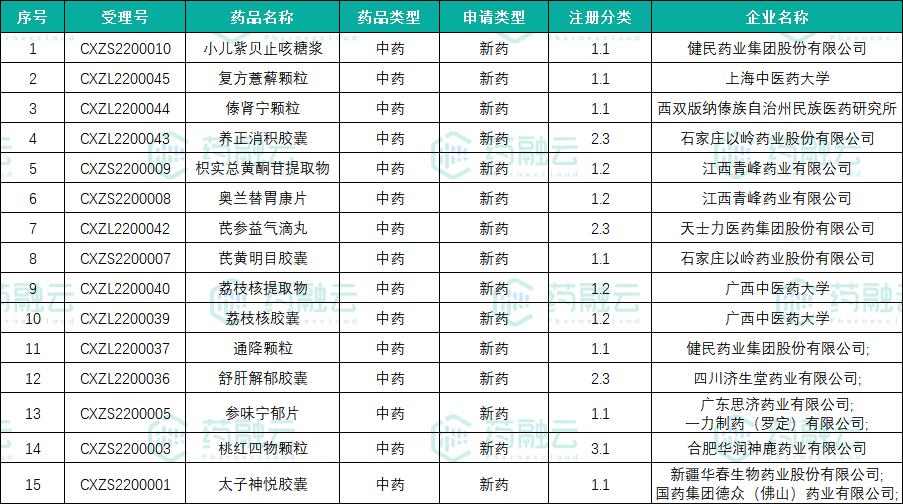
Catalogue of Acceptable Varieties of Innovative Chinese Medicine in 2022
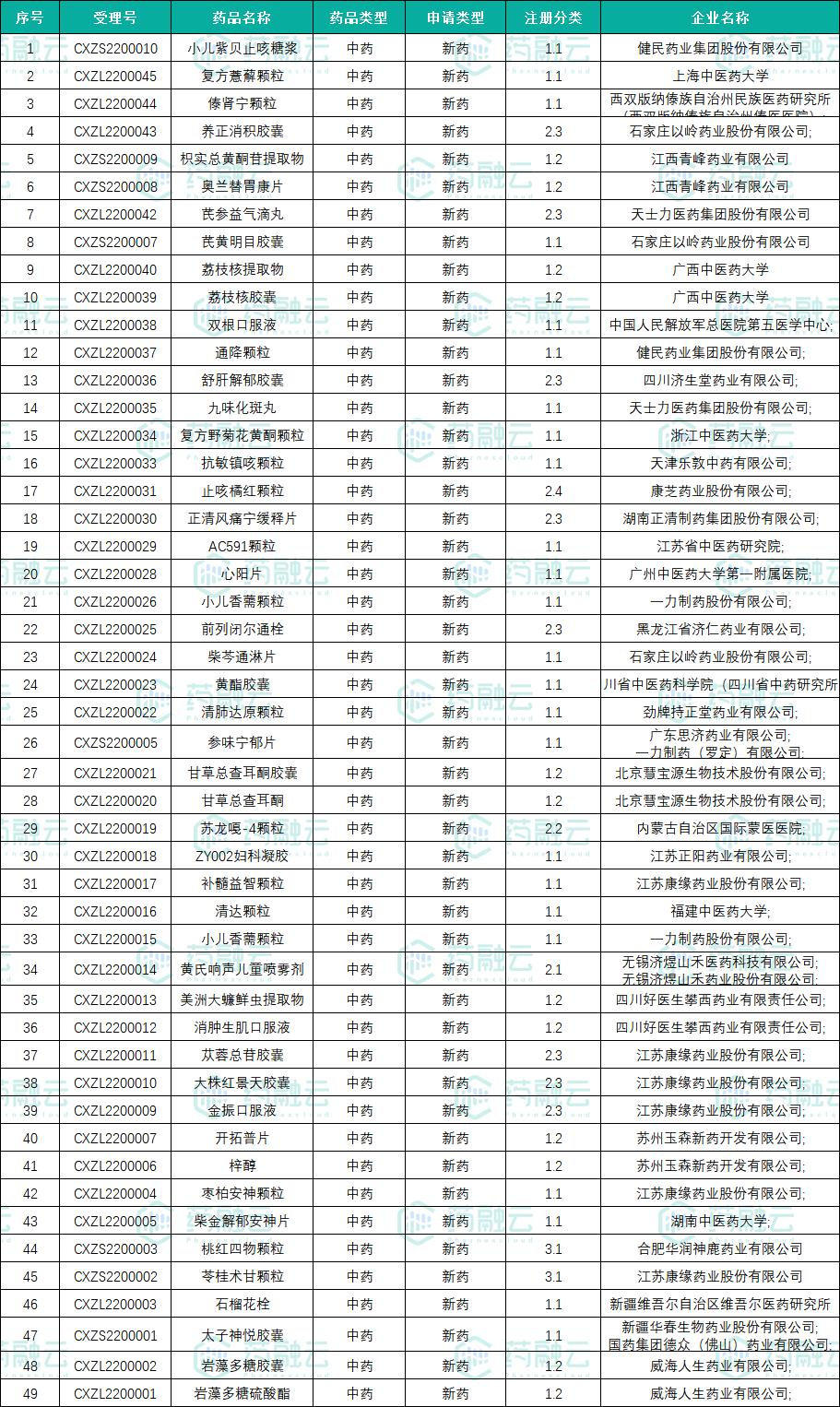
Finishing: Rong Yun.
According to the data of the National Bureau of Statistics, the total operating income of Chinese patent medicine production and Chinese herbal pieces processing in 2020 will exceed 600 billion yuan, and it is predicted that the market of Chinese medicine and related industries will approach 5 trillion yuan in 2030.
Traditional Chinese medicine (TCM) has made remarkable achievements in its inheritance and development for more than two thousand years. How to push the great wealth left by our ancestors to the world has always been the direction explored by generations. No matter how the times change, inheritance and innovation are always the two main lines of developing Chinese medicine.
I believe that in the next few years, with the continuous implementation of the basic policies for the innovation and development of traditional Chinese medicine, Chinese medicine enterprises will have the opportunity to resonate with the development of industries and policies. It is expected that in the future, innovative drugs of traditional Chinese medicine will go to a higher level on the innovation system that domestic western medicine has built, not only doing well in China, but also occupying a place in the world.
<END>